“ADHD is complex. There are no black and white answers.”
Attention deficit/hyperactivity disorder (ADHD) affects around five percent of children and adolescents worldwide. It is the most common psychiatric disorder in this age group. Adults are also affected. Whether at school, at work or in their personal lives, many people with ADHD suffer from the symptoms of their condition. They may struggle to sit still for long periods, have difficulty concentrating, or get into trouble due to their impulsivity. Many people with ADHD face challenges when it comes to reaching their full potential, despite often being highly intelligent. Yet realizing one’s potential is crucial for healthy personal development, especially in the early years. If this fails, there is a risk of setbacks: failure, frustration, bullying and even depression.
Delayed development of neurons
ADHD appears to be caused by a disruption in early neural development, the result of a complex interplay of genetic predispositions and environmental factors. In particular, the system responsible for executive function in the brain develops at a slower pace in individuals with ADHD. This means that brain areas such as the basal ganglia, are less active compared to people without ADHD. Dopamine, an important neurotransmitter colloquially known as the “happiness hormone”, plays a role in this brain region.
Edna Grünblatt, adjunct professor at the University of Zurich (UZH), has been researching ADHD for many years. Grünblatt leads research in the field of translational molecular psychiatry alongside Susanne Walitza, full professor and director of child and adolescent psychiatry and psychotherapy at UZH. Grünblatt explains: “ADHD is complex. There is a broad spectrum of types and manifestations. There are no black and white answers.”
The paradoxical effect of Ritalin
Ritalin is often the go-to medication when someone is diagnosed with ADHD. However, its mechanism of action is paradoxical: the active ingredient, methylphenidate, is a psychostimulant – meaning it typically has a stimulating effect. You might logically expect patients taking this drug to be more active. “In ADHD, however, the exact opposite happens. ADHD patients become calmer and more focused,” emphasizes Grünblatt. Methylphenidate increases the activity of neurons in the basal ganglia to approximately the level of individuals without ADHD. When executive function in the brain works properly, it leads to behavioral changes.
A different picture emerges when people without ADHD take Ritalin. In this case, the neurons in the basal ganglia decrease in activity, similar to untreated ADHD patients. The medication stimulates the neurons in the brain, making those who take it more restless and hyperactive. We know that methylphenidate works differently in ADHD. The question is, why?
Previously, researchers believed methylphenidate blocks dopamine transporters, resulting in more dopamine in the synapses, the point of contact between neurons. The theory was tested using genetically modified animals known as knockout mice – in this case mice lacking dopamine receptors, making them remarkably hyperactive. Surprisingly, they also became calmer when given methylphenidate. But how can the drug work if the dopamine docking sites are completely absent?
The central signaling pathway is key
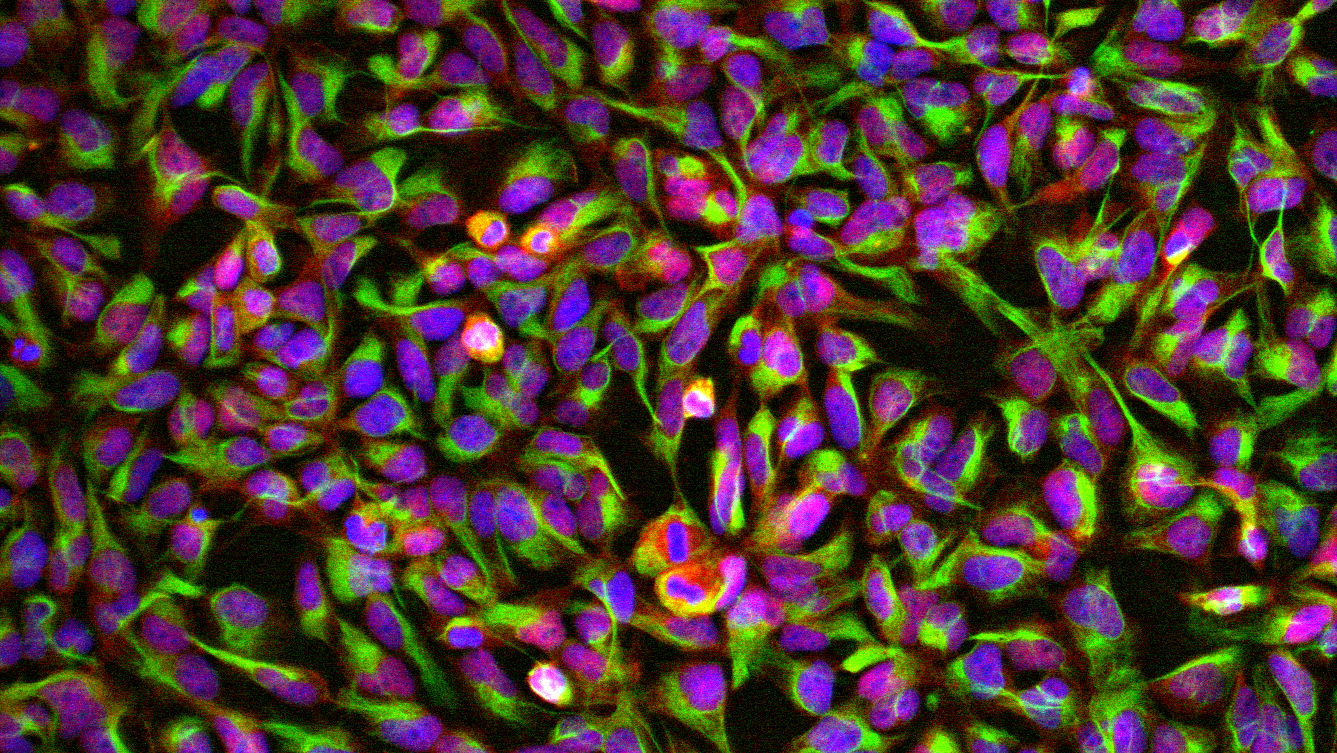
Methylphenidate must therefore work in a different way than previously thought – and this is precisely where Edna Grünblatt’s research comes in. Initially, she worked with neuronal cancer cells taken from both humans and animals. When methylphenidate was applied to these cell cultures, they began to divide more slowly. Instead of continuing to multiply, the cells began to differentiate and develop into neurons. The same occurred in embryonic stem cells from mice and rats, meaning the effect could not be attributed to properties specific to cancer cells.
This led the researchers to a new avenue of investigation: a central signaling pathway in the body that regulates cell division and development, known as Wnt pathway. Grünblatt and her team were able to demonstrate that methylphenidate does, in fact, activate the Wnt signaling pathway. This raised the next question: does methylphenidate also affect the neurons of ADHD patients via this mechanism?
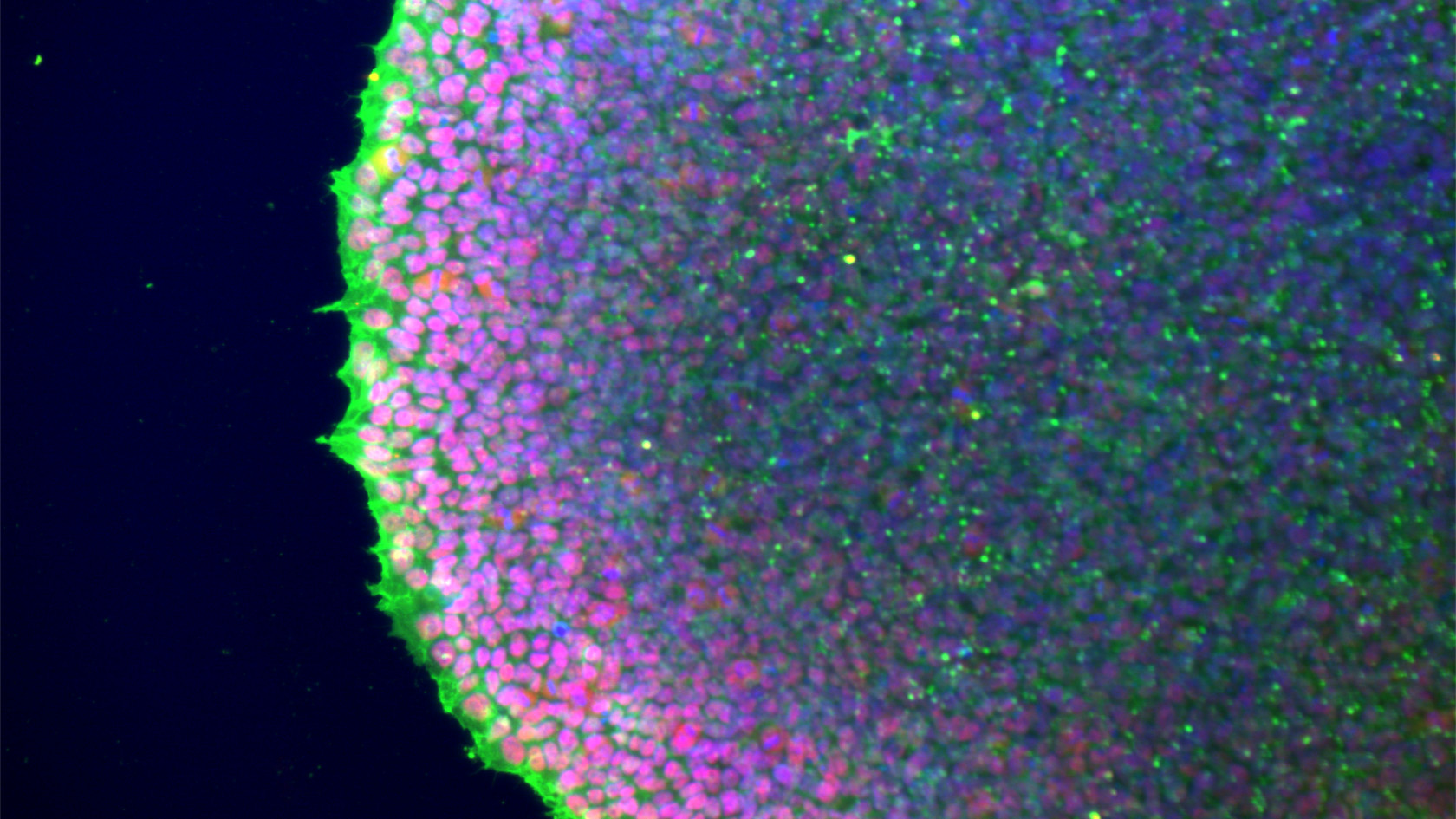
A crucial development in research opened doors for Grünblatt: induced pluripotent stem cells (iPSCs). These can be generated in the laboratory by reprogramming normal body cells back to an embryonic state using just four genes. These stem cells can then be used to grow different types of nerve cells.
From the clinic to the laboratory – and back again
“Conducting translational research is at the center of what we do. We want to understand what we see in the clinical setting at a molecular and cellular level,” says Grünblatt. Examining several iPSCs from ADHD patients presented the research team with yet another puzzle: in ADHD, the Wnt signaling pathway is already more active in the neuronal progenitor cells. “When we add methylphenidate to ADHD cells in a Petri dish, Wnt activity increases, even more compared to healthy neurons,” she adds.
In fact, in ADHD, neural progenitor cells grow more slowly in the iPSC models. If methylphenidate is administered, their division accelerates or normalizes. This appears to be because the Wnt signaling pathway is activated. When researchers block the pathway and apply the drug, this effect no longer occurs. Therefore, it is likely that Methylphenidate acts indirectly via Wnt. The effect of omega-3 fatty acids (fish oil) was also investigated, as some clinical studies have also demonstrated a positive effect. And indeed, the research team also found that they positively impact neural growth in ADHD iPSCs.
Tracking early development thanks to stem cells
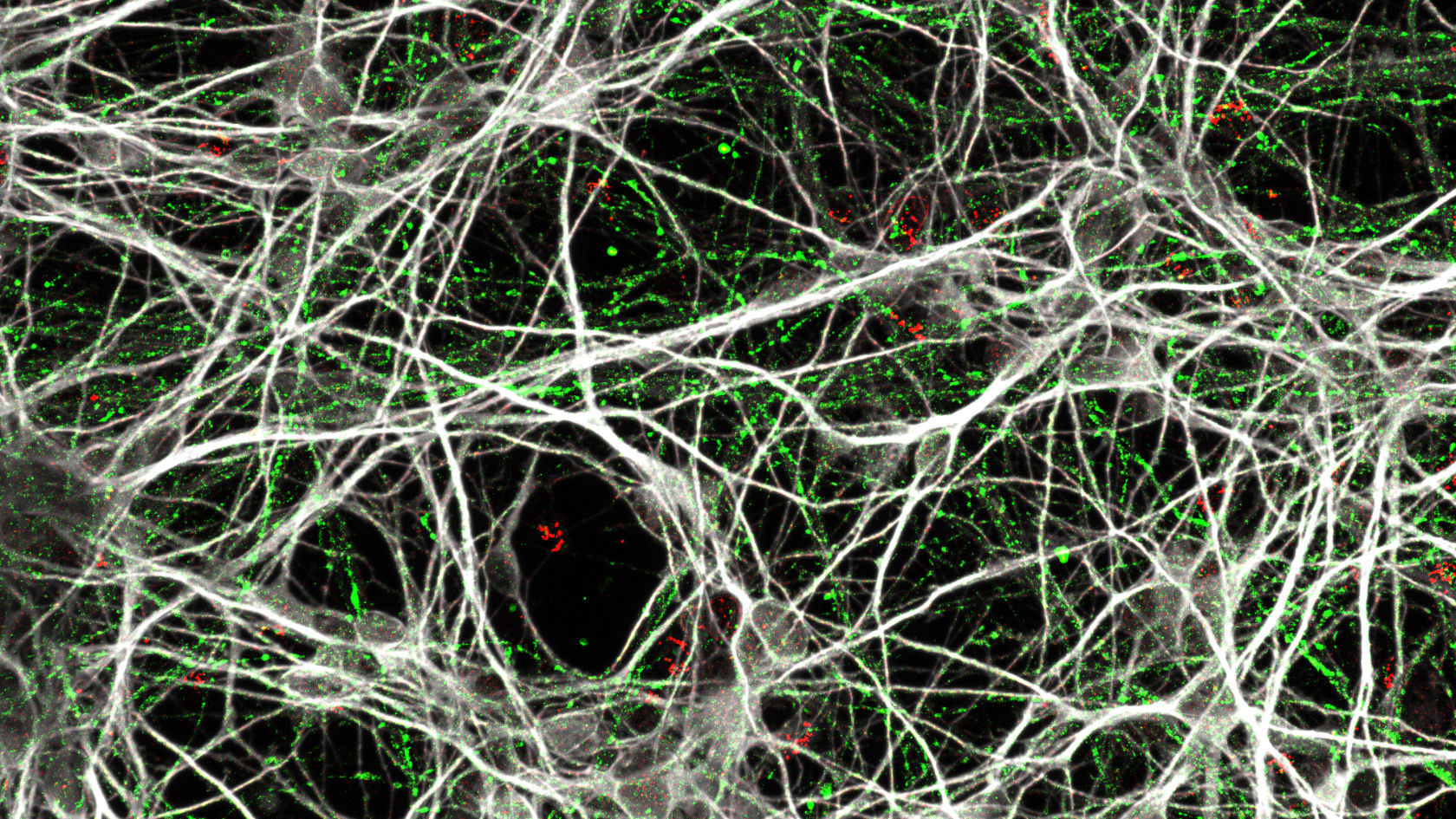
“Even though we’ve been using relatively simple 2D models consisting of just four or five different cell types, which come nowhere close to the complexity of the brain, we’ve already learnt a lot from them. This is because iPSCs carry the exact genetic material of our patients, which is very important in complex conditions such as ADHD,” emphasizes Edna Grünblatt. This means the development of neurons can be tracked over a long period of time, which is basically impossible to achieve in humans and embryos.
Grünblatt’s team used iPSCs from five ADHD patients and five control subjects for the measurements they have taken so far. Four additional cell lines were also used. The small number of cases is mainly due to the high costs involved in this type of research, as producing and handling the cells is complex. Nevertheless, induced pluripotent stem cells derived from patient cells have now become widely established as a model in biomedical research.
Grünblatt and her team are currently in a translational phase: they have already identified evidence of other molecular signaling pathways that are altered in ADHD, such as certain inflammatory pathways and oxidative stress. “In the long term, we hope to better understand risk factors for neurodevelopment in individuals with ADHD through our research – and possibly develop approaches for early prevention,” says the researcher.
Animal research is also necessary
While cell cultures are used in ADHD research, animals also play an important role. Grünblatt is working with two research teams in the Netherlands and Croatia who are conducting research into ADHD using animals. The focus is on behavioral measurements and the experiments involve the lowest levels of stress for the animals, corresponding to severity grade zero or one.
The advantage of animal research is that the complexity of the entire organism can be examined. This is important because the brain is in constant communication with the body; there is an interplay between the brain and hormones, the immune system and the microbiome in the gut. Factors such as stress and nutrition also play a role. Every model and method therefore contributes a piece to the puzzle that is ADHD.
Despite technical advances, animal experiments remain essential – especially for complex diseases such as ADHD. Modern animal research is based on the 3Rs principle of animal welfare: replacement, reduction and refinement. Many of the questions surrounding ADHD, such as those relating to cognition and behavior, can only be answered through research on living organisms. It is easy to determine whether an animal is hyperactive or impulsive, but this is simply not possible with cell cultures, or even with more complex organoids.