Live View: Stress-Induced Changes in Generations of Cancer Cells
Cells are the smallest units of life. But even within the same tissue or organ, they are not all identical. New variations arise continuously during cell proliferation. While genetic mutations alter the DNA sequence, epigenetic changes influence gene activity. The resulting cellular diversity is double-edged: On the one hand, heterogeneity aids development and adaptation to stress. On the other hand, it can lead to diseases such as cancer or reduce the effectiveness of therapies.
Tracking how cancer cells develop in real time
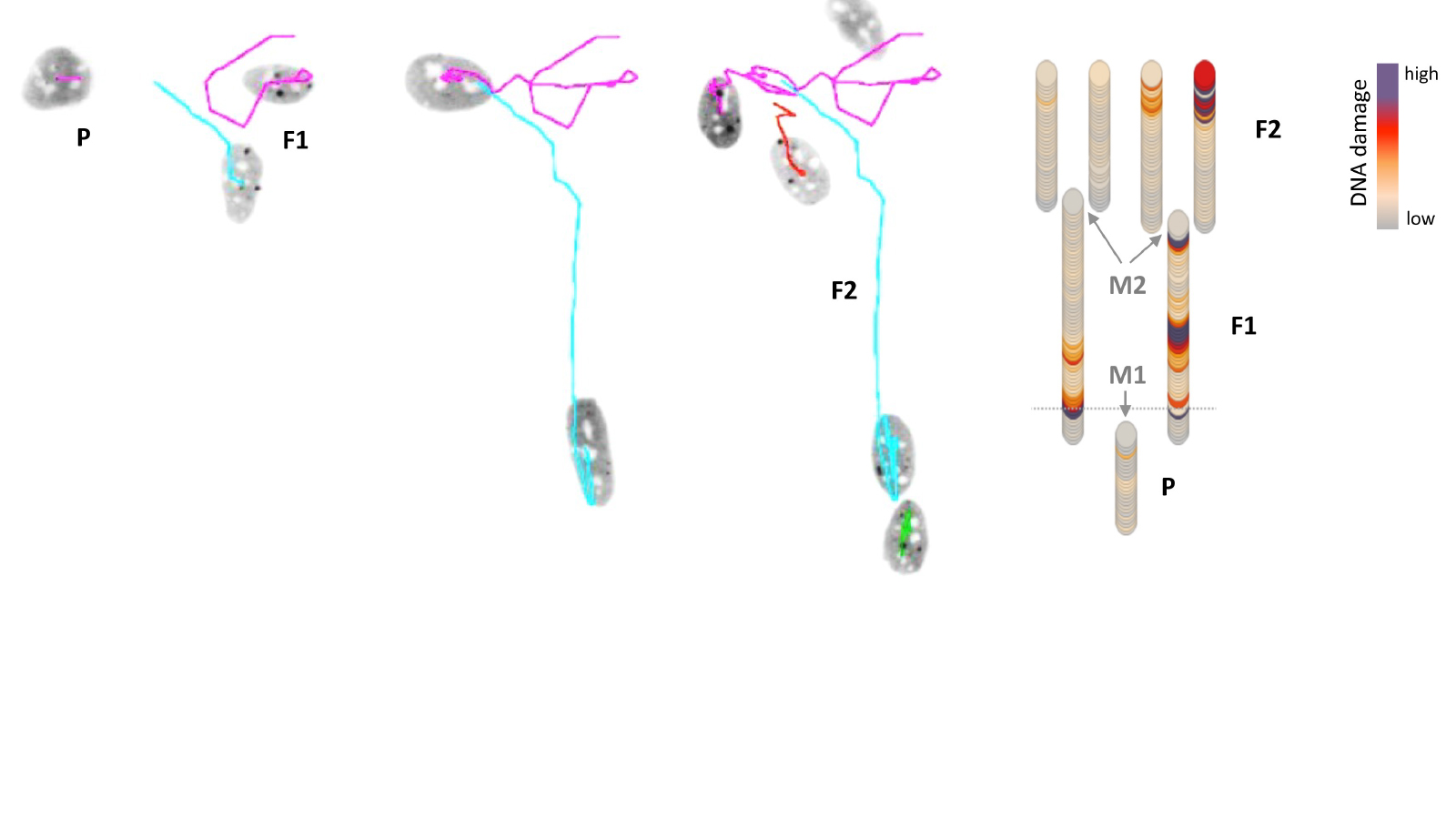
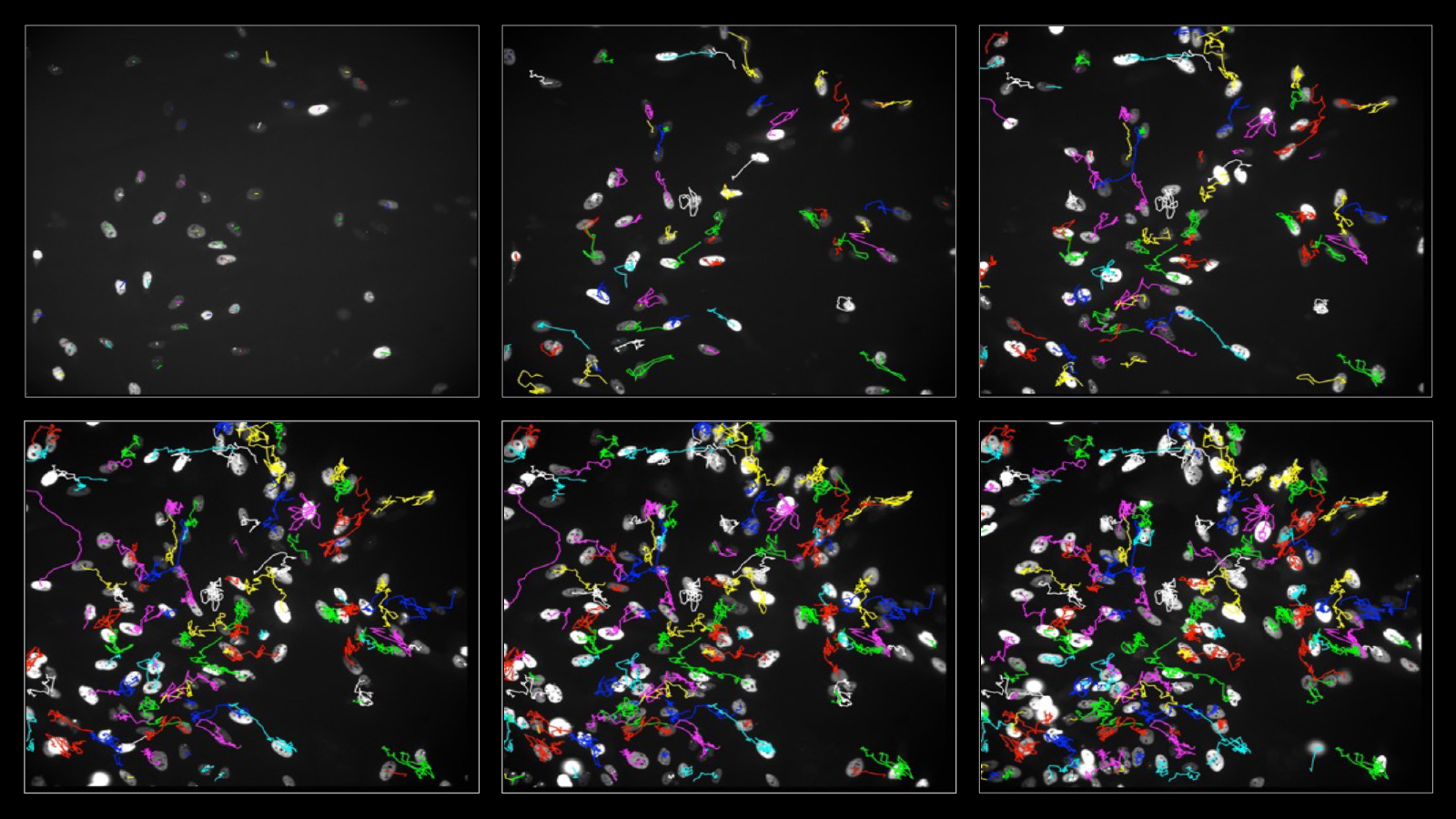
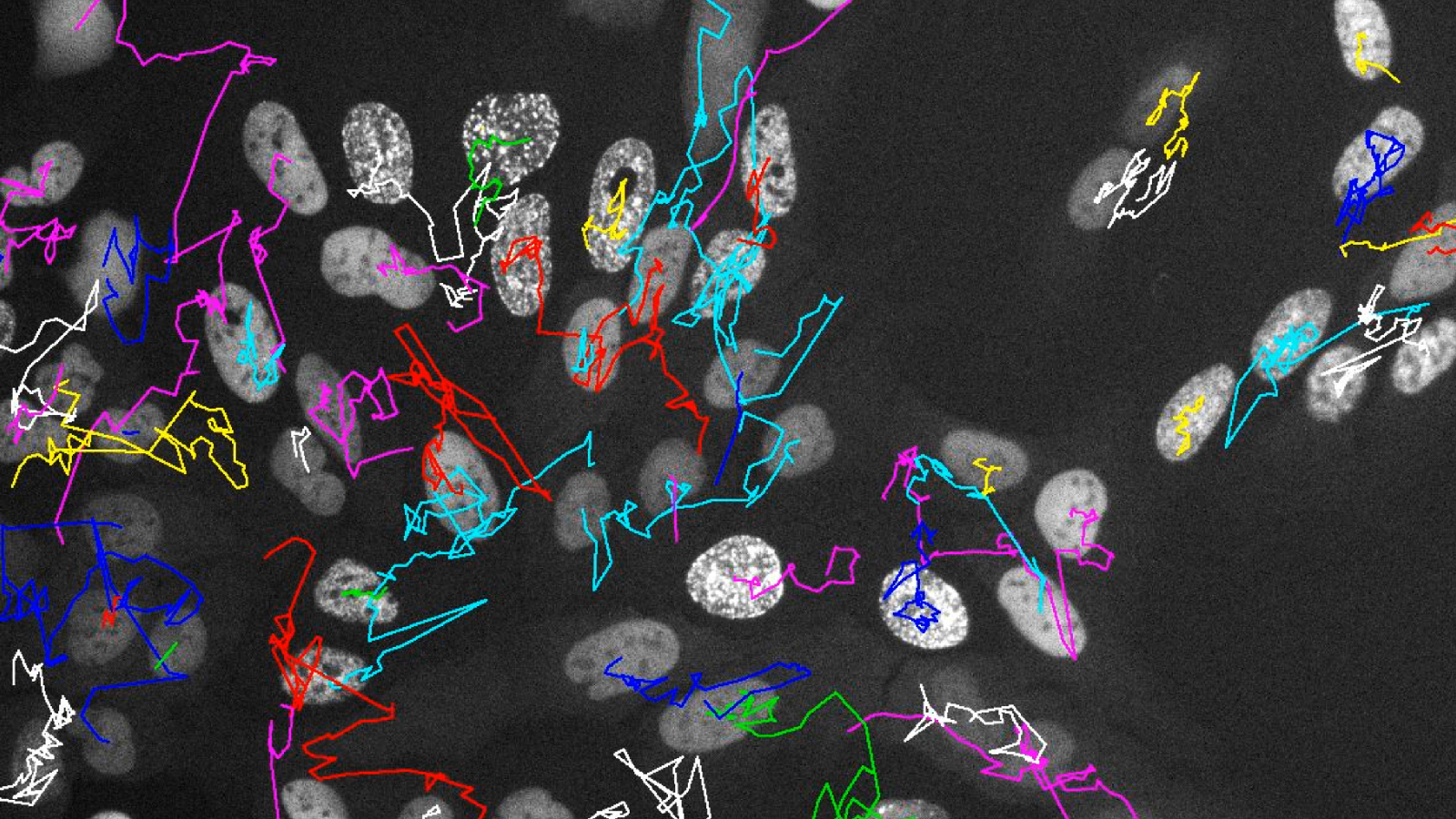
How these differences in the genome and in epigenetic control arise in cells, and how they are passed on to their daughter and granddaughter cells, has not yet been studied in detail. Now, researchers at the University of Zurich (UZH) have devised a method that allows them to track live under the microscope how cells develop and how cellular heterogeneity arises over several cell generations.
Using CRISPR-based genome editing, they attached fluorescent markers to two proteins: one to track the process of DNA replication and one to mark acquired DNA damage. “This allowed us to monitor over several cell generations how cancer cells respond to different stress factors and how this increases heterogeneity within the cell population,” says Merula Stout, UZH PhD student at the Department of Molecular Mechanisms of Disease and co-first author of the study.
Daughter cells vary significantly after stress
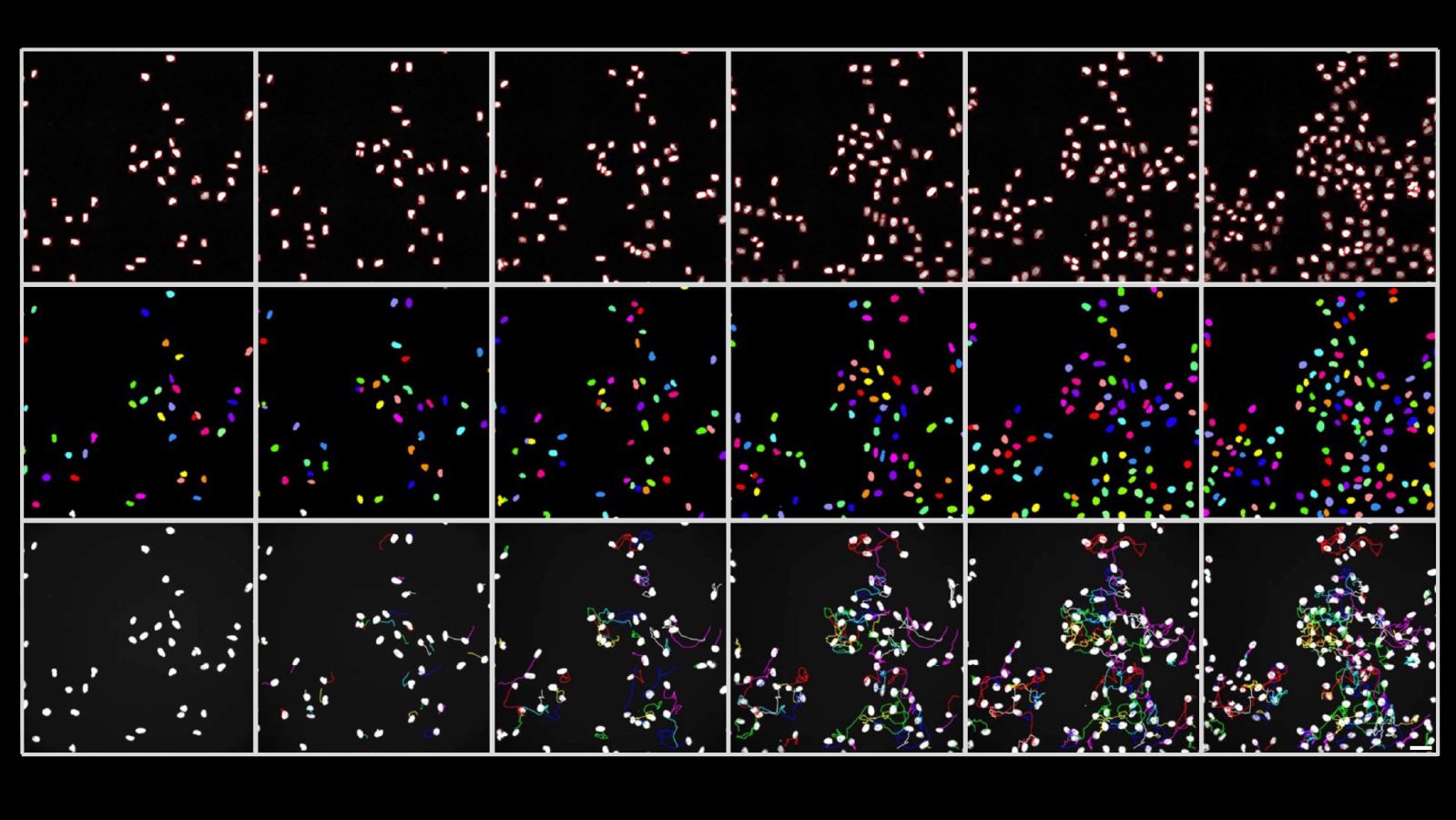
In addition to real-time measurements under the microscope, the researchers examined various endpoints, such as the strength of different stress signals in daughter and granddaughter cells. They then superimposed these measurements on the observed developmental trajectory of the same cells. “Using such cell family tree analyses, we were able to show that daughter cells no longer behave synchronously after cell division if the mother cell was exposed to stress,” says Stout.
According to the researcher, significant differences were found, for example, in the onset and duration of DNA replication and in the production of proteins that regulate the cell cycle. These differences continued in the next cell generation, thus increasing the heterogeneity in the cell population. DNA damage and stress therefore not only have short-term consequences, but also long-term effects on the diversity of cells.
Multiple genome copies promote resistance to therapy
Computer-assisted cell tracking also provided direct insights into how polyploidy arises in cells. In this process, cancer cells receive multiple copies of the genome. This in turn increases genetic complexity, allowing cells to adapt more quickly and develop resistance mechanisms against drugs.
The combination of real-time and endpoint measurements showed that different pathways towards polyploidy have different effects on the stability of the genome, thereby influencing the fitness of the cells. “We now have a better understanding of how cells with multiple copies of their genome develop. Potentially, our findings can be used to modulate the ways how polyploidy occurs and to better tailor therapies,” says UZH postdoc and co-first author Andreas Panagopoulos.
Just the tip of the iceberg
This study is the first to show in detail how different mechanisms influence genetic stability across multiple cell generations and increase heterogeneity between individual cells. The research team led by UZH professor Matthias Altmeyer aims to further develop and automate the method in collaboration with the technology platforms of UZH. “For research questions primarily concerned with single-cell and complex heterogeneity analyses rather than average effects, large amounts of data obtained in high throughput are required, and analyzing them may benefit from help by AI. It is very likely that we currently only see the proverbial tip of the iceberg,” says group leader Altmeyer.
Literature
Andreas Panagopoulos, Merula Stout et al. Multigenerational cell tracking of DNA replication and heritable DNA damage. Nature. 21 May 2025. DOI: https://doi.org/10.1038/s41586-025-08986-0
Contact
Prof. Dr. Matthias Altmeyer
Department of Molecular Mechanisms of Disease
Vetsuisse Faculty and Faculty of Science
University of Zurich
+41 44 635 54 91
E-mail