Navigation auf uzh.ch
Navigation auf uzh.ch
Martin Jinek is a master of understatement. When asked about his incredible series of publications in the most renowned scientific journals, the assistant professor at UZH’s Department of Biochemistry simply says, «That doesn’t happen very often.» The truth is that most research groups never succeed in doing what Jinek and his team have just done: A few days ago, he published an article in the British journal Nature, in March 2014, an article appeared in the American journal Science, and, shortly before that, Nature published the first of the three. In the world of sports, it would be a hat-trick.
There are two reasons for this exceptional run: First, Jinek is working on a «hot topic» and researchers around the globe have great expectations of it. His research matter – a protein called Cas9 – has the potential to revolutionize the field of genetic engineering. The second reason has to do with the expertise of Jinek’s team at UZH, which already enjoys an international reputation for its research into the structures of complex molecules.
A look at the history of this particular protein is necessary to fully appreciate the significance of their work. Although scientists succeeded in isolating Cas9 from bacteria just a few years ago, the origins of the research date back to the 1980s, when Japanese scientists discovered short segments of repetitive genetic building blocks (nucleotides) in the genome of bacteria that they called CRISPR (clustered regularly interspace short palindromic repeats). But, the function of these segments remained in the dark until, in 2005, several research teams recognized that CRISPR segments match with parts of the genomes of parasitic viruses .
Ensuing experiments confirmed the hypothesis that the CRISPR segments are part of a recognition system that bacteria use to identify harmful viruses – significant because identifying viruses is the first step to defending against them. In the next step, Cas9 takes over. With the help of a molecule (RNA) derived from the CRISPR sequence, the protein is led to the invading virus DNA and cuts it into pieces. Cas9 stands for CRISPR-associated protein number 9. «The molecule Cas9 is basically a multifunctional pair of scissors that can cut DNA», says Martin Jinek.
The discovery sent shock waves through the scientific community: It had become clear that what was previously viewed as a simple, unicellular organism had in fact a highly complex immune system. Jinek, who was then 28 years old, happened to be in the right place at the right time when the ground-breaking findings made the rounds: In 2007 the biochemist was working as a postdoctoral researcher in the laboratory of Jennifer Doudna at the University of California in Berkeley, where he was studying CRISPR and Cas9.
Because he had always been interested in the spatial structures of proteins and genome molecules, Jinek focused his efforts on the molecule in order to decode its workings. This marked the researcher’s entry into a field that has secured him and his team international recognition.
Martin Jinek is now working with his team to better understand how the protein functions on the molecular level. The most recent publication, based on the work of the team’s PhD candidates, Carolin Anders and Ole Niewöhner, and lab manager, Alessia Dürst, deals with the atomic details in the identification process between the DNA and the cutting protein. “We were able to show why individual nucleotides are needed to cut the DNA”, says Carolin Anders.
To obtain these findings, the PhD student had to crystallize variants of Cas9 – an extremely difficile task. After completing the experiments, she then x-rayed the crystals to create a high-resolution image of the molecule and its atoms. The images act as a kind of map that charts how the various molecular areas function.
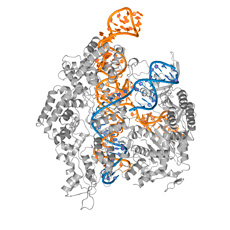
Two key ingredients needed to crystallize complex proteins like Cas9 are know-how and intuition. The success story includes work done at the Protein Crystallization Center at the UZH Department of Biochemistry and the Paul Scherrer Institute in Villigen, Aargau , which supplied the researchers with an extremely strong x-ray source called SLS. The «Swiss Light Source» enables the scientists to analyze small protein crystals. This procedure speeds up the experiments and increases productivity, which is evident in the astonishing series of publications.
With their work on structures, Martin Jinek and his team at UZH are engaging basic research. While exciting for experts in the field, the findings are not the only reason for the positive response from the most renowned scientific journals: Cas9 is widely believed to be heralding in a revolution in genetic engineering. «The molecule is the tool of the future for the high-precision modification of genome molecules», says Ole Niewöhner. By using the molecule, numerous, specifically targeted modifications can be made to the DNA of an organism in just one step.
Cas9 is seen as a promising tool for research and genetic engineering in areas such as stem cell research. Several groups are already using it, including developmental biologist Christian Mosimann at UZH. In the scientific journal «Science», Cas9’s diversity has already given rise to comparisons to a Swiss army knife.
Martin Jinek is laying the foundation for these applications. The main goal of the assistant professor from the Czech Republic who joined UZH last year is first and foremost understanding how this promising protein functions. In recognition of Jinek and his team’s great potential, the European Research Council (ERC) awarded them 1.5 million euro in 2013. The biochemist plans to use the funds to push forward in his research, the next step being a comparison of various representatives of different bacteria from the Cas9 family.
When discussing the value of the ERC grant, Martin Jinek remains true to the principle of understatement: «Thanks to the grant we can concentrate on the research, the objective being to stay competitive.»